Joining Thermal Management Graphite Composites
S-Bond® active solders enable graphite bonding and the joining of other carbon based materials to each other and to most metals within the constraints of thermal expansion mismatch. S-Bond® alloys have active elements such as titanium and cerium added to Sn-Ag, Sn-In-Ag, and Sn-Bi alloys to create a solder that can be reacted directly with the carbon surfaces prior to bonding using specialized S-Bond® treatments for solder joining. Reliable joints have been made between graphite and carbon based materials with all metals including steel, stainless steels, titanium, nickel alloys, copper and aluminum alloys.
In high power density electronics, there is a need to rapidly spread and dissipate heat generated by the high frequency operations in the electronics. In order to improve the heat dissipation capacity of graphite based materials, Applied Nanotech developed a new passive thermal management material, CarbAl™, which is a carbon-based material with a unique combination of low density, high thermal diffusivity, and low coefficient of thermal expansion based on Figure 1.
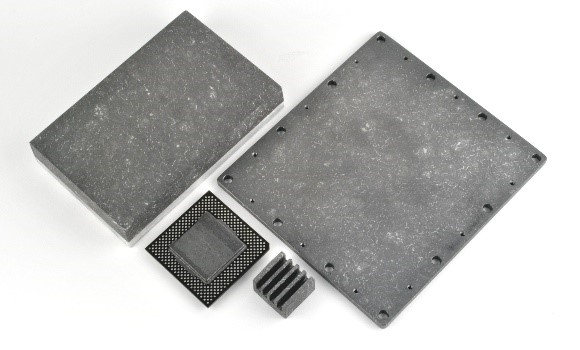
Figure 1. Picture of the CarbAl-G high thermal diffusivity graphite composite.
Applied Nanotech reports that CarbAl™ has a density of 1.75 g/cm3 compared to 2.7 g/cm3 for aluminum and 8.9 g/cm3 for copper. While copper has a slightly higher thermal conductivity than CarbAl™, 390 W/mK compared to 350 W/mK, CarbAl’s thermal diffusivity is approximately 2.9 cm2/sec compared to 0.84 cm2/sec for aluminum and 1.12 cm2/sec for copper.
S-Bond Technologies and Applied Nanotech have collaborated to make heat spreaders with CarbAl-G cores combined with copper and aluminum claddings to make the heat spreaders that are more robust and able to be fabricated to support a high density of high power electronic devices, yet be mounted in standard “PC” card configurations as seen in Figure 2.

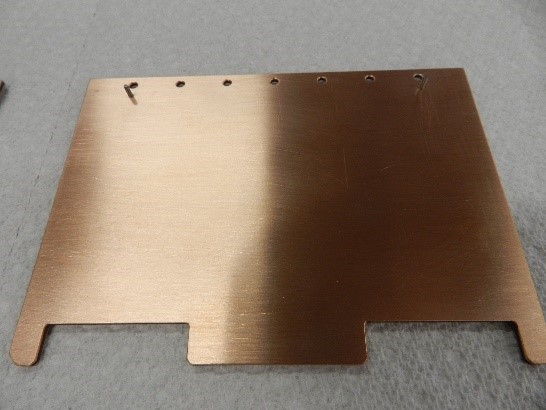
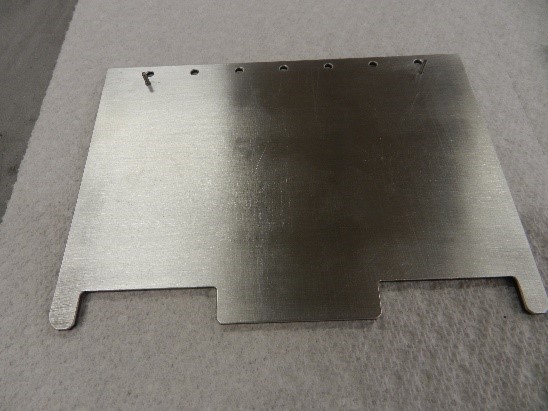 Figure 2. Copper and Aluminum clad CarbAl-G circuit boards.
Figure 2. Copper and Aluminum clad CarbAl-G circuit boards.
These clad CarbAl-G cored boards have utilized active S-Bond® solder layers to intimately bond and thermally connect the thin copper or aluminum claddings to the lightweight, high thermal diffusivity CarbAl-G composite sheets.
S-Bond® CarbAl-G bonding and joining was thermally activated using S-Bond Technologies proprietary process, which prepared the CarbAl-G surfaces and developed a chemical bond to the surface, through reactions of the active elements in S-Bond® alloy to the graphite in CarbAl-G. These joints start with processing the graphite/carbon surfaces at elevated temperatures in a protective atmosphere furnace with S-Bond® alloy placed on the graphite-carbon surfaces to be joined. At these elevated temperatures, the active elements in S-Bond® (Ti, Ce, etc.) react with the ceramic to develop a chemical bond. After the CarbAl-G is prepared / S-Bond® metallized, the CarbAl-G is then S-Bond® soldered to the aluminum or copper sheets to for a metal clad CarbAl-G composite plate that can then be machined into a heat sink plate to which high power electronic devices be mounted.
The S-Bond® solder joints produced:
- Are ductile, based on Sn-Ag or Sn-In alloys
- Exceed the strength of the CarbAl-G
- Are thermally conductive, with S-Bond® alloys having k = 50 W/(m-K)
- Are metallic and this electrically conductive with a metallurgical bond
S-Bond Technologies has developed extensive experience in active, S-Bond® solder joining of graphite, carbon and carbide to metals. Contact Us to evaluate our joining solutions for your graphite joining applications. For more information on CarbAl-G, please contact Applied Nanotech. Inc









 Carbon/graphite compressor seal rings are employed in many compressors and more and more metal backed graphite seals are being used in higher efficiency compressors. Frictional heating of seals can degrade metal backed graphite seals, therefore good thermal contact between the graphite seal ring and the metal backing is needed to improve cooling of the seal.
Carbon/graphite compressor seal rings are employed in many compressors and more and more metal backed graphite seals are being used in higher efficiency compressors. Frictional heating of seals can degrade metal backed graphite seals, therefore good thermal contact between the graphite seal ring and the metal backing is needed to improve cooling of the seal. 




 S-Bond Technologies’ active solder joining solutions have been used by by Fermi National Accelerator Laboratory for joining carbon:carbon and pyrolytic graphite in its particle accelerator program. The improved Forward Particle Detector (FPIX) is to be used in Compact Muon Solenoid (CMS) and used for high-resolution, 3D tracking points, essential for pattern recognition and precise vertexing, all embedded in a hostile radiation environment.
S-Bond Technologies’ active solder joining solutions have been used by by Fermi National Accelerator Laboratory for joining carbon:carbon and pyrolytic graphite in its particle accelerator program. The improved Forward Particle Detector (FPIX) is to be used in Compact Muon Solenoid (CMS) and used for high-resolution, 3D tracking points, essential for pattern recognition and precise vertexing, all embedded in a hostile radiation environment.
 where the blades were inserted, Figure 3. After metallization, the blade and nozzle segments were inserted into a heated alignment press, Figure 4. After heating and insertion, the nozzle segment/ TPG blade assembly was cooled and removed. Figures 1a – b, above, showed the final fully bonded assembly.
where the blades were inserted, Figure 3. After metallization, the blade and nozzle segments were inserted into a heated alignment press, Figure 4. After heating and insertion, the nozzle segment/ TPG blade assembly was cooled and removed. Figures 1a – b, above, showed the final fully bonded assembly.



